Screening anticancer drugs
The success rate of drug development is reported to be as low as about 10% overall, or about 5% for anticancer drugs(1). Moreover, the development of anticancer drugs involves a period of 10 years or longer from target identification to market launch and massive costs ranging from several billion yen to tens of billions of yen(2). This is why means of increasing the success rate of anticancer drug development have been sought in order to provide anticancer drugs with confirmed efficacy and safety to patients more rapidly.
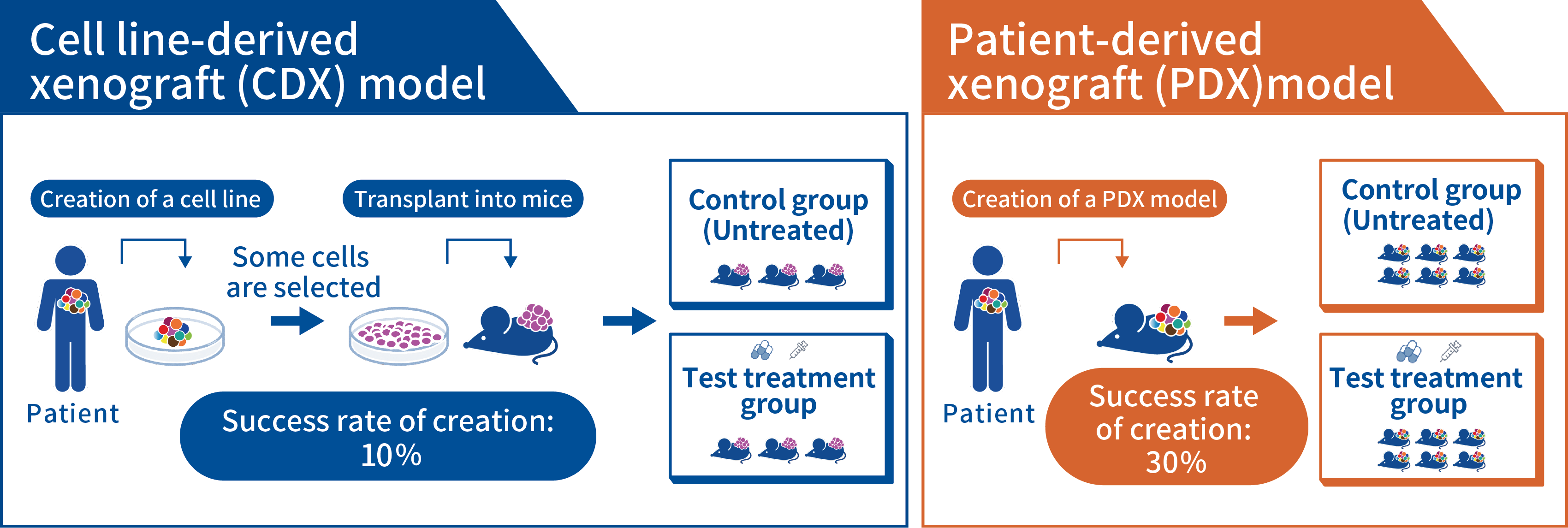
Screening of anticancer drugs has been conducted using mouse tumor cell lines such as L1210 and P388 since the 1950s and human cell lines such as those in the NCI-60 panel created by the National Cancer Institute (NCI) in the US since the 1990s(3, 4). In vitro drug sensitivity testing using cell lines and in vivo drug sensitivity testing using cell line-derived xenografts (CDX) models in mice have been used as standard methods of assessment, and drugs with confirmed efficacy in preclinical studies have progressed to clinical trials. Even if, however, screening using cell lines determines that anticancer drugs are efficacious, a small proportion actually display efficacy in cancer patients. The following factors are considered to be the main reasons for this discrepancy in efficacy between preclinical and clinical studies.
- The heterogeneity of a patient’s tumor is lost during the creation of a cell line and only some of the cell populations that tend to grow are concentrated (loss of heterogeneity).
- The tumor microenvironment is lost during the creation of a cell line and interaction between the tumor and microenvironment is lost (loss of the microenvironment).
- The cell line lacks the three-dimensional structure of the patient’s tumor and the histology of the CDX differs from that of the patient’s tumor (changes in histology).
- Due to a loss of heterogeneity, only some of the cell-specific genetic abnormalities in the patient’s tumor are taken over in the cell line (change in genetic abnormalities).
Due to these limitations of discovery and development using cell lines and CDX models, a pressing issue is to devise systems of screening that can better predict the efficacy of anticancer drugs in actual patients (i.e., a system with substantial ability to predict clinical efficacy).
- [References]
- (1). Mullard A. Parsing clinical success rates. Nature Reviews Drug Discovery. 2016;15(7):447-.
- (2). Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nature Reviews Drug Discovery. 2010;9(3):203-14.
- (3). Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84(10):1424-31.
- (4). Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66(7):3351-4, discussion 4.
What is a PDX?
Over the past few years, patient-derived xenograft (PDX) models have garnered attention as a platform for screening with substantial ability to predict clinical efficacy. A PDX is a cancer model that is created by transplanting patient tumor tissue directly into highly immunodeficient mice without any culturing. The PDX model is extremely simple and has long been reported as a concept, but its practicality has been increased by the development of highly immunodeficient mice. Immunodeficient mice began with the development of nude mice in the 1960s. In the 2000s, NOG mice (NOD.Cg-PrkdcscidIl2rgtm1Sug/ShiJic) were created by the Central Institute for Experimental Animals and NSG mice (NOD.Cg-Cg-PrkdcscidIl2rgtm1Wjl/SzJ) were created by the Jackson Laboratory. The development of these combined immunodeficient mice has increased the success rate of PDX creation and enhanced their utility as a platform for screening anticancer drugs.
Notable features of PDX models are that they retain the heterogeneity, microenvironment, and histology of the patient’s tumor. Moreover, the ability of PDX models to predict clinical efficacy is reported to be 30-80%, which is higher than that of other platforms for screening. PDX models are expected to be used as a screening platform in drug discovery and development(5-7). A disadvantage of a PDX is the cost and time required for preparation and screening. Immunodeficient mice are invariably required for a PDX, so PDX models cost more than cell culture media used to create cell lines. In addition, a long period of time is required from the transplantation of the patient’s tumor into mice until the establishment of the PDX via passaging (generally, a PDX is considered stable after 3 passages), so this increases the personnel expenses to maintain those models.
- [References]
- (5). Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nat Rev Cancer. 2015;15(5):311-6.
- (6). Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318-25.
- (7). Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998-1013.
(Figure1)Comparison of CDX models and PDX models
| CDX model | PDX model | ||
|---|---|---|---|
| Preparation process | Success rate of creation / maintenance | Approx. 10% / simple and inexpensive | Approx. 30% / complicated but effective |
| Tumor characteristics | Difference in structure with respect to the original tumor | Changes in the microenvironment and histology | Retains the microenvironment and histology |
| Genetic information | Only partially retained | Mostly retained | |
| Drug efficacy testing | Period from preparation to conclusion | ▼ Brief (1-2 months) | ▲ Prolonged (3-12 months) |
| Cost | ▼Low | ▲High | |
| Tumor heterogeneity | ▼Low (homogeneous) | ▲Maintained (heterogeneous) | |
| Drug dose | Administered at the maximum tolerated dose for mice | Yet to be determined | |
| Ability to predict clinical efficacy | ▼Approx. 5% | ▲30-80%? |
The current status of PDX libraries in other countries
In the US and Europe, universities, government agencies, and commercial organizations have been constructing PDX libraries and using them to screen anticancer drugs since the 2010s. In the US, the National Cancer Institute (NCI) in particular took the major step of transition from screening anticancer drugs with its NCI-60 panel to PDX models in 2015, and it created the PDX Network (PDX-Net), which includes universities and other organizations(8). In Europe, EurOPDX was launched in 2013 as a consortium of EU countries, and it has constructed a PDX library with more than 1,500 models to date(8). In addition, several contract research organizations (CROs) in Europe, the US, and China are currently constructing PDX libraries to screen anticancer drugs.
In Japan, the National Institutes of Biomedical Innovation, Health, and Nutrition (NIBIOHN) have long focused on the usefulness of PDX models, and the Institutes have been working on the creation of immunodeficient mice and PDX models(9). In addition, Fukushima Medical University has constructed the Fukushima PDX (F-PDX) as a project to create a support site for the pharmaceutical industry in Fukushima (10).
However, there are fewer PDX models in Japan than in the US and Europe, and that number needs to be increase. In addition, PDX models in Europe and the US are mainly created using surgical specimens of early-stage cancers. In order to use PDX models to develop anticancer drugs, PDX models that reflect conditions in patients with advanced or recurrent cancer who are actually administered anticancer drugs are needed. Moreover, there are racial differences in genetic alterations in tumors, so a PDX library of various types of cancer found in Japanese patients is needed to accelerate the development of anticancer drugs in Japan(11).
The J-PDX Library and its features
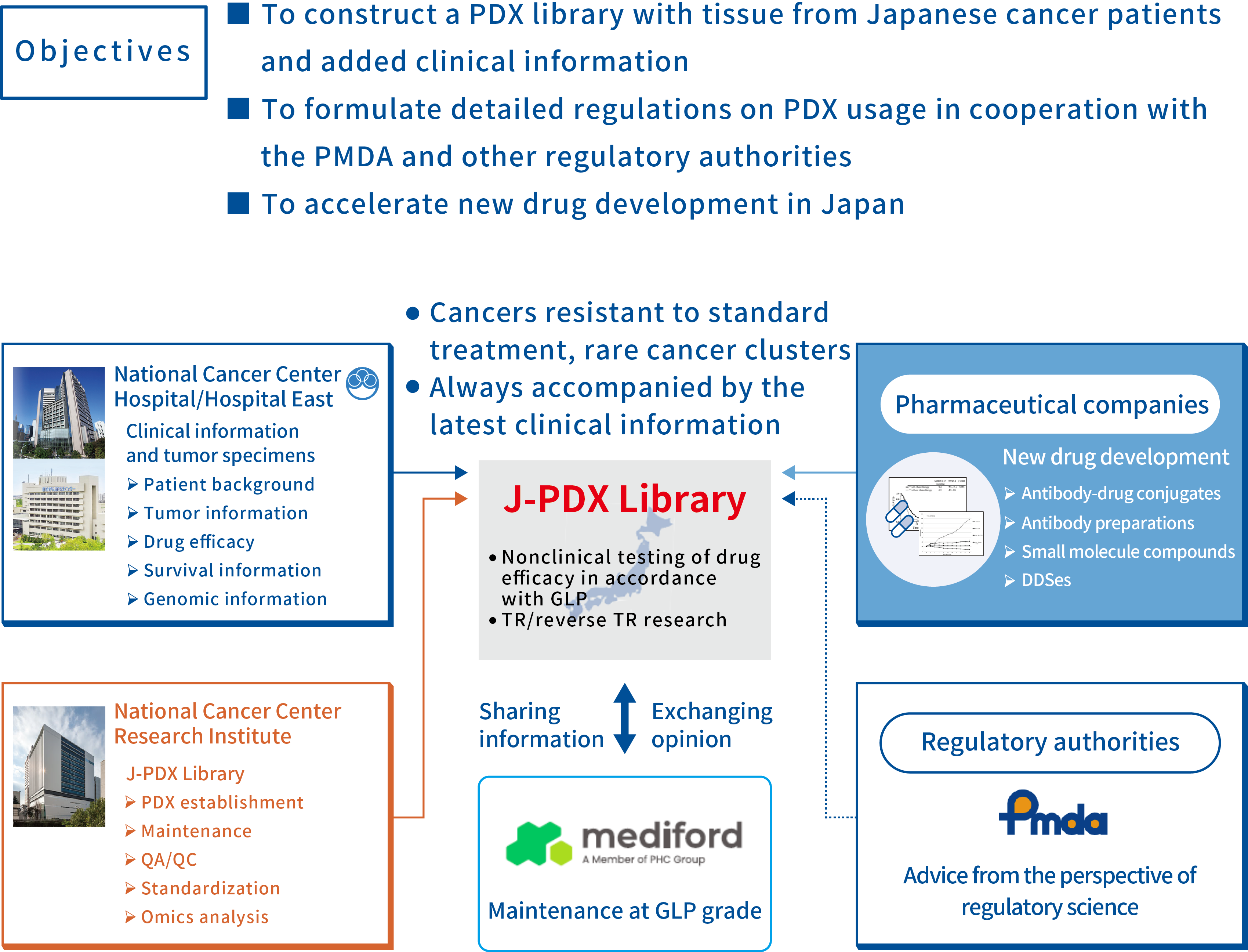
Within this context, the National Cancer Center, with the support of the Cyclic Innovation for Clinical Empowerment (CiCLE) project of the Japan Agency for Medical Research and Development (AMED), started to construct the J-PDX Library in 2018 in collaboration with the LSI Medience Corporation(now Mediford Corporation*) as part of the Project to Construct a PDX Library with Tissue from Japanese Cancer Patients to Promote Cancer Treatment(12). The J-PDX Library focuses on advanced or recurrent cancers and rare cancers for which anticancer drugs are difficult to develop, and it is also actively creating PDX models from biopsy specimens of tumors that have acquired resistance to standard treatment. The Library is adding detailed clinical information to each model. As of the end of October 2022, more than 1,700 specimens were registered and more than 500 models were engrafted, making the J-PDX Library the largest such library in Japan (Figure 2, Table 1).
*LSI Medience Inc. has transferred its business to Mediford Corporation effective November 1, 2023.
- [References]
- (8). Meehan TF, Conte N, Goldstein T, Inghirami G, Murakami MA, Brabetz S, et al. PDX-MI: Minimal Information for Patient-Derived Tumor Xenograft Models. Cancer Res. 2017;77(21):e62-e6.
- (9). https://ahmhcdd.nibiohn.go.jp
- (10). https://www.fmu.ac.jp/home/trc/provision/f-pdx/
- (11). Saito M, Shiraishi K, Kunitoh H, Takenoshita S, Yokota J, Kohno T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107(6):713-20.
- (12). Yagishita S, Kato K, Takahashi M, Imai T, Yatabe Y, Kuwata T, et al. Characterization of the large-scale Japanese patient-derived xenograft (J-PDX) library. Cancer Sci. 2021;112(6):2454-66.
(Figure2) J-PDX Library Project
(Table1)Features of the J-PDX Library
| J-PDX Library | PDX libraries in other countries | |
|---|---|---|
| Stage | Advanced/recurrent cancers,rare cancers | Mainly early-stage cancers |
| Patients | Patients at the NCC | Assembled from other facilities |
| Tumor specimens | Biopsy specimens/surgical specimens | Mainly surgical specimens |
| Previous medical treatment | Mainly cancers resistant to standard treatment | Mainly untreated cancers |
| Consent obtained | Thoroughly obtained | May not be obtained in some instances? |
| Clinical information | Previous medical treatment Efficacy PFS/OS Results of genetic analysis, etc. (Clinical information is updated as needed) |
Limited information |
| QA/QC for creation of PDX models | Compliance with GLP | Mainly at the lab level |
Mission of the J-PDX Library
The mission of the J-PDX Library is to accelerate drug discovery and development in Japan in order to rapidly provide more efficacious anticancer drugs to cancer patients. In addition, the Library will continue to identify issues and create a system for the research use of PDX models so that many researchers can reliably use the valuable samples and information provided by patients without wasting them.
Issues in drug discovery and development using PDX models
PDX models should serve as a research platform to accelerate drug discovery and development. However, PDX models are relatively new bioresource, so numerous issues, such as quality control, views on their handling, and methods of use in preclinical studies, have yet to be fully resolved. Thus, the National Cancer Center is conducting “A Study on the Identification and Standardization of Issues in Drug Discovery and Development Using PDX models” with the support of Research on Regulatory Science for Pharmaceuticals and Medical Devices of the Japan Agency for Medical Research and Development (AMED).
The following three issues are the focus of this study.
- Identifying ethical, legal, and social issues (ELSI) associated with the proper use of a new bioresource, i.e., PDX models
- Examination of methods of quality control during PDX creation
- Collection of information and the exchange of opinions required to standardize methods of conducting preclinical studies using PDX models
In the course of the study, opinions regarding PDX models will be sought from the following relevant parties, research institutes, organizations, and companies.
- Patients, families, and patient groups who donate specimens for PDX models
- Research institutes and commercial organizations that create PDX models
- Institutions supplying the laboratory animals used to create PDX models
- Contract research organizations to assess drugs using PDX models
- Pharmaceutical companies and industry associations commissioning assessments using PDX models
- Regulators that interpret and evaluate the data obtained from PDX models
- Research institutes and researchers conducting research using PDX models
As the study proceeds, we intend to discuss matters and exchange opinions on an individual basis, so we ask for your cooperation.
