Steps in the development of anticancer drugs
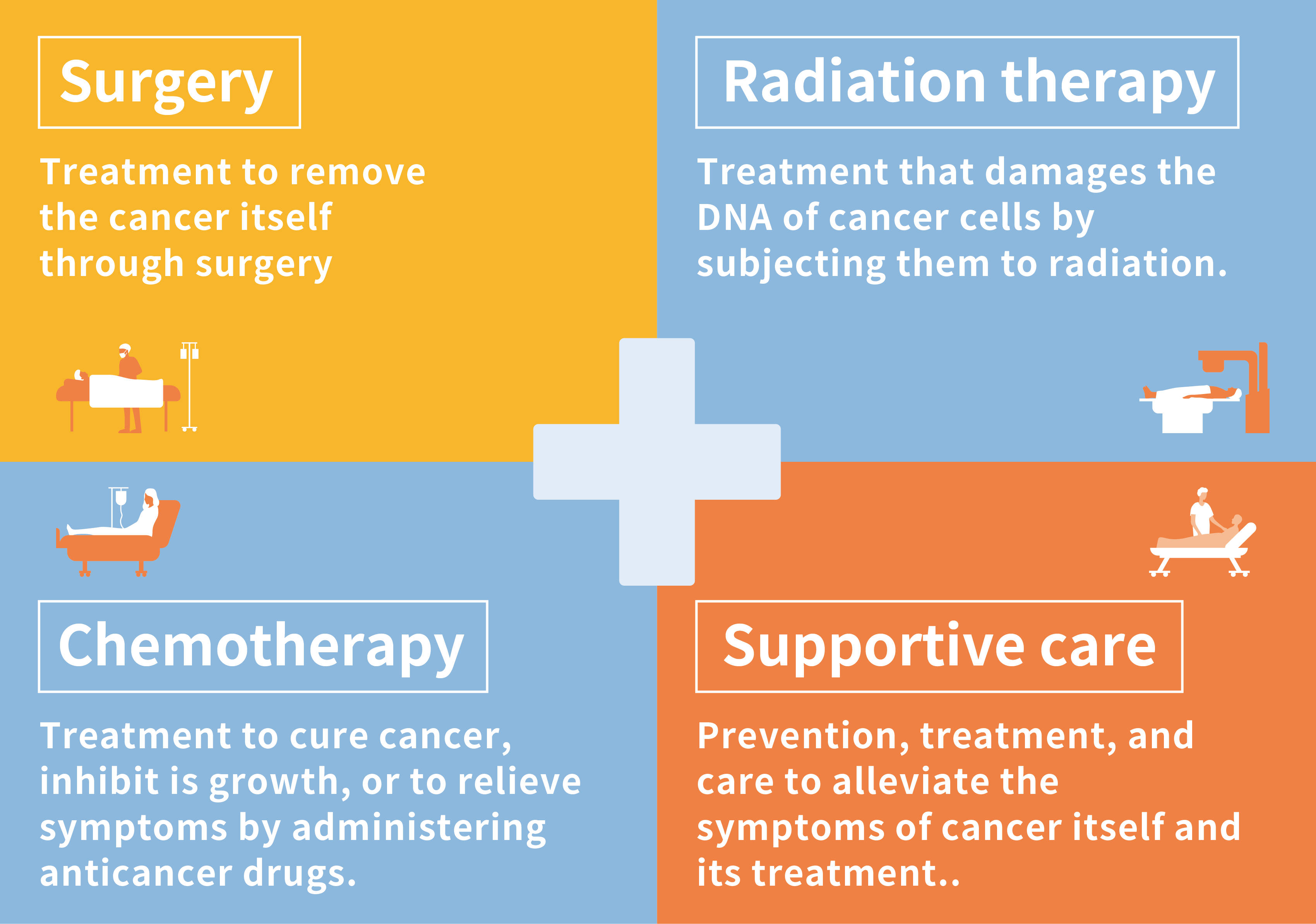
Anticancer drugs are used to cure cancer, inhibit its progression, or to relieve the physical symptoms it causes. There are 4 pillars of cancer treatment: surgery, radiation therapy, chemotherapy (here, treatment with anticancer drugs is collectively referred to as chemotherapy), and supportive care. Anticancer drugs play a role in one of those pillars.
The process of making (developing) anticancer drugs can be roughly divided into “preclinical studies” and “clinical trials. Preclinical studies are conducted in cells or animals before administering an anticancer drug to humans, and clinical trials are conducted afterwards to test whether the drug causes no serious side reactions in humans and whether it is efficacious.
In preclinical studies, research is conducted to determine what sort of targets an anticancer drug should, what sort of anticancer drugs can be expected to be efficacious, what sort of adverse reactions will occur when the drug is administered to animals, what will happen if the drug is administered for a long time, and how the anticancer drug disappears from the animal’s body. In order to fully confirm the safety of a drug when administered to humans (adverse reactions) in a preclinical study, experiments using cells are conducted and then experiments are conducted in which the drug is administered to mice, dogs, or monkeys in a step-by-step process. A long and deliberate study is conducted to confirm whether the anticancer drug should really advance to a clinical trial or not.
Anticancer drugs that are confirmed to be safe in cells and laboratory animals and that are expected to be efficacious proceed to a clinical trial. A clinical trial is roughly divided into 3 phases: phase I, phase II, and phase III. A phase I trial is the first human trial in which an anticancer drug is administered to a small number of patients. The dose is gradually increased to confirm its safety, and the dose of the anticancer drug to administer in the subsequent trial (Phase II, Recommended Phase II Dose; RP2D) is determined. In a phase II trial, based on the RPD2 determined in the phase I trial, the anticancer drug is administered to a larger number of patients to confirm its safety and to assess its efficacy. If the efficacy of the anticancer drug is seen in the phase II trial, it proceeds to a phase III trial to confirm its efficacy in a larger number of patients and to assess whether the anticancer drug is more efficacious than standard treatment. If the efficacy of the anticancer drug is confirmed in the phase 3 trial, an application for approval is submitted to the regulatory authorities in each country or region (in Japan, the Pharmaceuticals and Medical Devices Agency; PMDA).
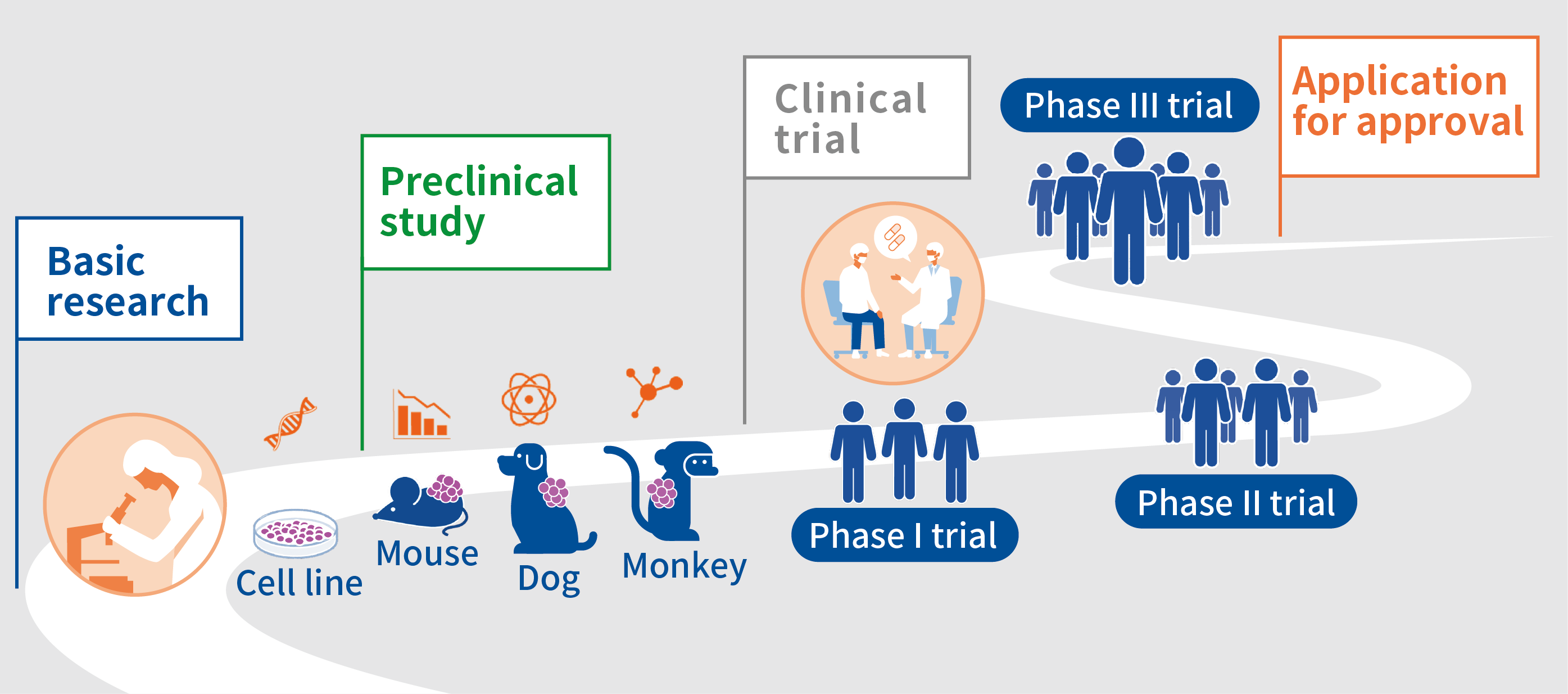
An anticancer drug takes a long time to be approved after preclinical and clinical trials, and the economic cost of development ranges from 10 billion yen to 100 billion yen. Moreover, the probability of an anticancer drug being successfully developed and approved is very low, i.e., around 5%. In other words, if 100 anticancer drugs are developed, 95 of them will not be approved due to problems with their safety or efficacy. This is why efforts are being made around the world to develop safer and more efficacious anticancer drugs more rapidly and to provide them to patients.
Why developing anticancer drugs is so difficult
Presumably, there are various reasons why developing anticancer drugs is so difficult. One is the stage in which to assess “what sort of anticancer drug can be expected to be efficacious” in preclinical studies.
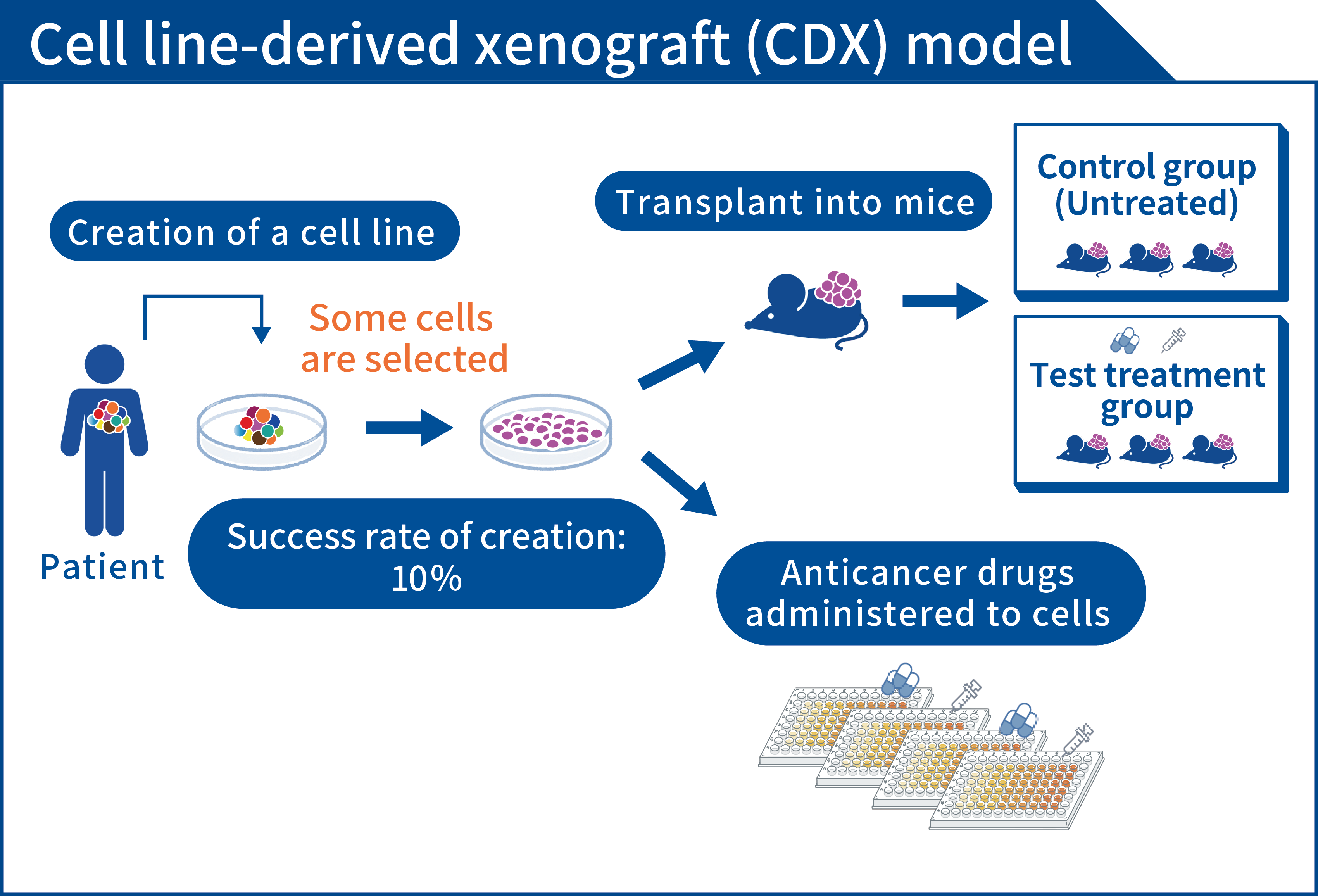
Cancer cell lines can be grown on a laboratory bench. In the past, these models have been used to assess the efficacy of anticancer drugs. Cancer cell lines are produced by growing cancer tissue from patients together with culture media in a dish. Since the 1990s, 60 cancer cell lines have been created by the National Cancer Institute (NCI) in the US and used in the NCI-60 panel, and several cancer cell lines have also been created in Japan. New anticancer drugs are administered to these cancer cell lines and the efficacy of each drug is assessed. Many highly efficacious anticancer drugs have progressed from preclinical studies to clinical trials and have ultimately been approved.
However, cancer cell lines are artificially cultured in a two-dimensional dish. A disadvantage of this system is that “cancer tissue in a patient’s body is not reproduced as-is.” Each cancer cell in a patient’s body has slightly different aspects, and this characteristic of cancer is known as “heterogeneity.” This heterogeneity causes cancer cells to move to various organs (metastasis) and to cause anticancer drugs to cease being efficacious. In cancer cell lines, heterogeneity is lost in the process of artificially culturing cancer cells in a two-dimensional dish, and only cancer cells that readily grow in the dish may remain. Thus, the selection of an anticancer drug using a cancer cell line may result in the selection of an anticancer drug that is not efficacious against heterogeneous cancer cells in the patient’s body.
A new assessment model for development of anticancer drugs
Given these circumstances, preclinical studies should assess the efficacy of anticancer drugs against “cancer with the same heterogeneity as in the patient’s body.” Over the past few years, “three-dimensional cultures” and “patient-derived xenograft (PDX models)” have garnered attention as models that can be used to assess cancer in the same way as in a patient’s body.
In contrast to cancer cell lines that have been cultured in a flat (two-dimensional) dish as a model for assessment, three-dimensional culture is a model in which cancer cells can grow through use of a special culture plate and culture medium. Cancer cells in three-dimensional culture grow in the same manner as cancer cells in the patient’s body and have similar structures and functions, so this model can reproduce conditions in vivo in a relatively simple manner in a culture plate. A three-dimensional culture ultimately grows cancer cells on a culture plate, so one of the issues with that approach is that it has difficulty reproducing the blood vessels that nourish the cancer and the cells around the cancer (immune cells and various cells called fibroblasts that are collectively referred to as “stroma”).
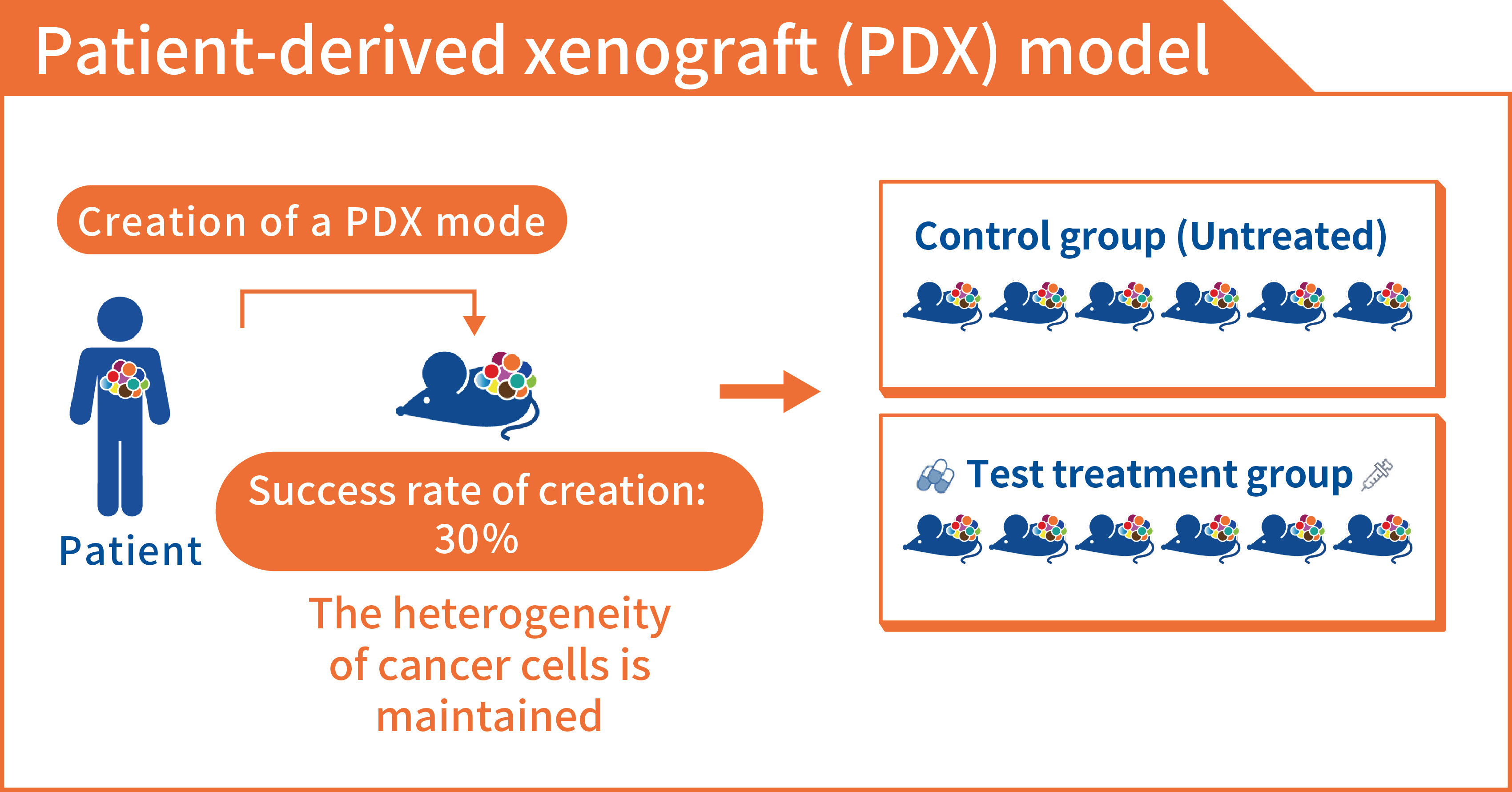
A PDX is a model in which cancer cells from a patient are transplanted into laboratory animals (mainly severe combined immunodeficient mice are used), and the cancer cells grow in the body of the mice. Although PDX models have long been considered as a model, the immune cells of mice attack human cancer cells when they are transplanted into mice, so growing cancer cells in mice has been difficult. Since the 2000s, however, the Central Institute for Experimental Animals in Japan and the Jackson Laboratory in the US have developed severe combined immunodeficient mice, enabling the creation of PDX models. An advantage of a PDX model is as it grows cancer cells in a mouse’s body, the blood vessels and nutrients in the mouse’s body can be made to resemble those in the human body, and the structure and functions of cancer tissue are similar to those in the human body. In addition, even if the stroma surrounding the cancer is replaced by mouse cells, the stroma is considered to be similar to human cancer tissue. The most promising aspect of those models is that the results of those assessments are reported to coincide with the results of administering those drugs to patients at a very high rate of 30-80%. Since the probability that an anticancer drug developed using conventional cancer cell lines is approved is about 5%, PDX models are expected to be models that can predict the efficacy of anticancer drugs in patients with a very high level of accuracy.
The PDX library with tissue from Japanese cancer patients (J-PDX Library)
The National Cancer Center has been promoting the development of anticancer drugs using PDX models in order to promote the development of anticancer drugs in Japan and to provide more efficacious anticancer drugs to patients more rapidly. We have been developing a platform for research and development of anticancer drugs using PDX models. We receive cancer tissue samples from patients visiting the National Cancer Center Hospital and Hospital East and create PDX models. Since 2018, we have been creating a “PDX library with tissue from Japanese cancer patients (the J-PDX Library)” as a system for use in researching and developing anticancer drugs.
How the J-PDX Library is used
A PDX is used in studies to assess the efficacy of anticancer drugs, so it is an important platform for promoting the development of anticancer drugs. Moreover, PDX models retain the heterogeneity of cancer, so they are expected to be used in research to ascertain the true nature of cancer as a disease, such as how cancer it originates, how it metastasizes, and how anticancer drugs become inefficacious.
The National Cancer Center’s mission is “to provide optimal cancer care to the public in cooperation with society.” We will operate the J-PDX Library so that PDX models created with the cooperation of patients can be used to provide optimal cancer care. The J-PDX Library is used by many universities, research institutes, and pharmaceutical companies.
Samples and information received from patients
The J-PDX Library creates PDX models from the tumor tissues received from patients and it collects clinical information (age, sex, the type of cancer, stage, type of treatment, its effectiveness, its duration, laboratory results, etc.) from the patients’ medical records to create a database.
During the development of anticancer drugs, important questions are to identify “patients who are more likely to benefit from the drug,” i.e., which type of cancer the drug will be efficacious against, whether the drug is efficacious against cancers with genetic abnormalities, and whether the drug will be efficacious after a certain type of treatment. This can increase the likelihood of providing the best treatment to each patient. Similarly, groups of patients who are expected to benefit from an anticancer drug that is being developed are also determined in clinical trials based on the results of preclinical studies. Therefore, clinical information on each patient is a very important clue when developing anticancer drugs.
In the J-PDX library, the efficacy of an anticancer drug is evaluated in a preclinical study using a PDX model and its evaluation is based on the clinical information on the patient who has donated tumor tissues to create that PDX model. This should presumably allow the identification of groups of patients in whom the drug will be efficacious prior to or early in a clinical trial. Clinical information on patients is assessed along with the results of studies using PDX models. This should accelerate the development of anticancer drugs and provide new anticancer drugs to patients more quickly.
Clinical information on patients contains very important personal information that may identify the patient from whom a PDX was created. This is why the National Cancer Center and users of the J-PDX Library handle personal information with strict care to prevent its inadvertent disclosure. In specific terms, the cancer cells and information we receive are anonymized and managed using an identification number (an encrypted number) so that individuals cannot be identified. When information is provided to institutions conducting joint research, personal information will be managed pursuant to an agreement ensuring that said information is fully protected.
Withdrawal of consent
Patients who cooperate with the J-PDX Library may withdraw their consent at any time even if they have already consented to participate in a study. If a patient withdraws consent, the patient’s cancer cells and PDX models will be rendered into a state where the individual cannot be identified and then discarded. Stored unused samples can be destroyed, but if samples have already been used in research when consent is withdrawn, some of the research data may have already been disclosed or shared. In general, data (including research data) cannot be completely discarded. PDX models created from the patient’s cancer cells and tumors created from the PDX models (research products) cannot be discarded if they have already been provided to researchers outside the National Cancer Center. If those items cannot be discarded, the record (known as “a correspondence table”) linking PDX models created from the patient’s cancer cells, research products, or information to information that can identify an individual, such as the patient’s full name and medical record number, will be discarded, thus severing the link to the individual patient.
